Some time ago, top tennis player Alexander Zverev, who lives with Type 1 Diabetes, was reprimanded for taking his insulin injection on court. Officials told him he could only do it twice per match — and only in the restroom. 🤦🏻♂️
The global diabetes community, including us, reacted with thousands of emails — and the decision was quickly reversed.
Once again, we proved that none of us are alone, no matter how successful or wealthy we are. 💙
Zverev Signs with Medtronic! 😊
This story didn’t go unnoticed by Medtronic.
Just like the rest of the world, they saw Zverev using basic disposable insulin pens — and decided to offer him a partnership.
Besides insulin pumps, Medtronic also makes the world’s most advanced smart insulin pens. These pens connect via mobile app to Medtronic’s CGM sensors — currently the Guardian 4 and Simplera, and soon the new Instinct sensor, which lasts 15 days and is extremely small.
The connected app will remind you if you forget a dose, show your insulin-on-board (IOB), provide smart insights for better glucose control, and much more.
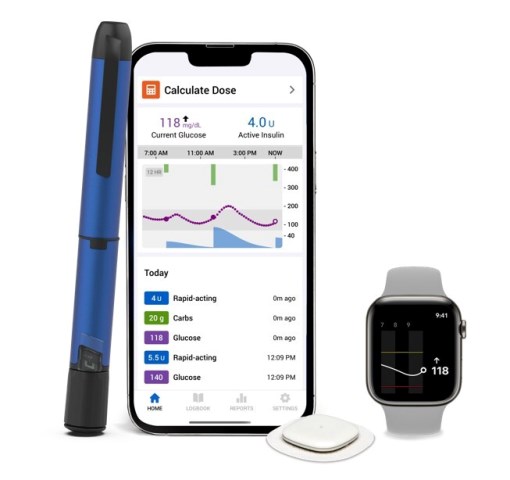
Medtronic InPen™ smart insulin pen with Simplera™ CGM
Medtronic 780G and the New Instinct Sensor!
One of the hottest topics at the EASD 2025 Conference in Vienna was the brand-new Instinct sensor, which lasts 15 days and will be manufactured for Medtronic by Abbott.
At first, the news spread like wildfire: the Instinct + 780G system had received FDA approval in the U.S. But no one knew when people would actually start using it.
That changed when the first Medtronic 780G users in the U.S. received official emails confirming they could start ordering the new Instinct sensors — and that shipments would begin within a month! 😊
Turn up your speakers and watch Medtronic’s fantastic new MiniMed video!
What About Europe?
The good news: EU regulatory approval is much easier to obtain than in the U.S. 😊
That’s probably why Ohad Cohen, one of Medtronic’s global leaders, told attendees at EASD Vienna:

What Else Did Medtronic Announce About the 780G?
As expected, Medtronic presented new studies showing that the MiniMed 780G system significantly improves outcomes across a wide range of people with diabetes — from children as young as two, to women with Type 1 Diabetes during pregnancy, and even adults with Type 2 Diabetes.
In Croatia, the 780G is currently approved for children aged 7 and older, but based on these findings, broader access is expected soon. 😊
Conclusion
Technology that can dramatically improve the lives of people with diabetes already exists — the challenge now is to make it accessible to more people.
Medtronic recently revealed that over 140,000 people participated in their 780G clinical studies, meaning the total number of users is even higher!
Abbott, on the other hand, announced that more than 6 million people are currently using their CGM sensors.
In other words — Abbott clearly has the capacity to produce enough Instinct sensors for Medtronic, and users of the MiniMed 780G pump won’t be waiting long. 😊
Here’s to progress — and to better days for all of us living with diabetes. 💙






